A new method of sampling secretion from the nose and paranasal sinuses
A new method of sampling secretion from the nose and paranasal sinuses
Vladimir S. Kozlov, Larisa L. Derzhavina and Larisa V. Kuptzova Rhinological Centre 'YARTEC', Yaroslavl Regional Hospital, Yaroslavl, Russia
Introduction
A considerable number of methods exist for sampling secretion from the nasal cavity for subsequent biochemical and immunological examination'-4. It is also known that usually both nasal mucosa and that of the paranasal sinuses (PNS) are involved in a pathological process. Sampling secretion from PNS applying non-invasive methods has proved to be rather difficult. For the last 14 years, we have been successfully applying sinus-catheters 'YAMIK' in the treatment of sinusitis which allow atraumatically to evacuate secretion from the nose and PNS5>6.
The present paper is aimed at finding out whether the use of the ' YAMIK-3' sinus catheter is effective for sampling secretion which will then be biochemically tested.
Material and methods
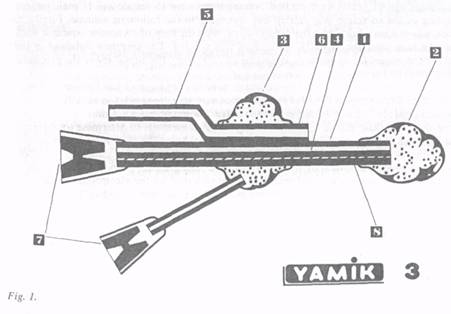
Figure 1 is a diagram of the 'YAMIK-3' model sinus catheter. It consists of a body (1) with a passage (4) inside for inflating a vessel (2) at the side of the body. There is also a flexible rod (8) in the body able to modify the body to fit the nasal cavity anatomy. Movable with regard to the body, there is an inflatable cuff (3) with a tube (5) rigidly attached to the cuff and having a working passage (6). The passages designed for inflating the vessels (2) and the cuff (3) are provided with valves (7).
Operating principles
After anesthesia of the nasal mucosa, the sinus catheter is inserted into the nasal cavity. The vessel (2) is inflated in the rhinopharynx and choane while the cuff (3) is inflated in the vestibule of the nose. The nasal cavity is sealed off from both sides, thereby making a closed space inside. A syringe is connected to the working passage allowing the creation of negative and positive pressure (+15-20 mbar) in the nasal cavity and PNS. When negative pressure is created, the pathological secretion can be evacuated out of the PNS through the natural ostia.
Twenty-six patients with a diagnosis of acute purulent sinusitis, with ages ranging from 16, to 58 (average age 41 years) were studied. Among them were 15 female and 11 male patients.
Sampling of the secretion was carried out according to the following scheme. Firstly, the secretion was evacuated during a front rhinoscopy, with the help of a vacuum aspirator with a fine tip. An hour later the 'YAMIK' procedure was applied. The secretion obtained in both cases was centrifuged for 15 minutes at the rate of 2000 rpm. The liquid above the precipitate was used for examination.
The amount of secretion obtained by the vacuum-aspiration method was between 0.5 and 2 ml, the average amount being 1.2 + 0.3 ml. The amount of secretion obtained with the 'YAMIK' method was between 0.5 and 10 ml, with an average amount of 6.3 ±1.4 ml.
Above-the-precipitate liquid was tested using standard methods to determine total protein, protein fractions, the lactate dehydrogenase activity level being the most informative tests for characterizing the pathological processes7.
Total protein was determined by the Lowry method8 using an SF-46 spectrophotometer.
The protein fractions in the nasal secretion were obtained by the electrophoresis division method on the films made of acetate celluloid with the use of veronal-medinal buffer pH 8.6.
Densitometry was performed by analyzing electrophoresis pictures with the AP-901 'Uk-raina'.
The activity level of lactate dehydrogenase was determined by an optical test with the use of chemicals (LABSISTEME Company) and the biochemical analyzer FP-901 (Finland).
Results and discussion
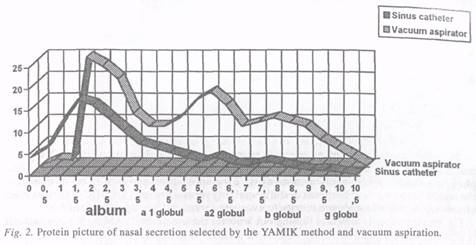
Table 1 shows the results of examination of the nasal secretion obtained by the two different methods.
In our opinion, the considerably higher protein concentration in the secretion obtained with the help of the 'YAMIK' sinus catheter can be accounted for by the fact that the creation of negative pressure resulted in stimulation of the processes of active transudation out of the capillary network and inter-tissue space in the nasal cavity and pathologically affected sinuses. This is proved by a considerably higher percentage and absolute content of albumins in the secretion obtained with the 'YAMIK' method. The controlled changeable pressure does not increase destructive changes in the nasal and PNS mucosa. This can be judged by the lack of increase in the lactate dehydrogenase activity level as an indicator of cell destruction.
There is no considerable qualitative difference in the protein picture of the nasal secretion in either sampling case.
Table 1. Examination results of nasal secretion
|
Biochemical parameters
|
Nasal secretion indices
|
|
YAMIK method
n = 26
|
Vacuum aspirator
n = 26
|
P
|
|
Total protein
(Lowry's method)
% Index albumin/globulin Activity LDG Ed/L
LDG activity
per 1 g protein
|
33.8±1.0
65+6/35±1.2
1880±120 55±5.1
|
8.4±0.8
42±1.8/58±2.1
1937±100 230+18.0
|
p<0.05
p<0.05 p>0.05
p<0.05
|
Conclusions
From analyzing the data obtained, the use of the 'YAMIK' sinus catheter can be considered an effective method of sampling secretion from the nasal cavity and PNS. The biological liquid obtained includes components in the concentration suitable for biochemical and immunological examination. There is also enough liquid to carry out the examinations. The liquid obtained does not need diluting or concentration, thus providing reliable results.
References
1.Portenko GM: Method of sampling secretion out of the nasal cavity. J ENT Dis 1:82-83, 1988
2. Prozorovskaya KN, Shevrygin BV, Tarasova GD, Karpova EF, Lyalina DV: Humoral immunity in children with vasomotor rhinitis. Vestnik Otorhinolaryngol 5:11-13, 1988
3. Naclerio RM: The role of chemical mediators in allergic rhinitis. Am J Rhinol 7:4-149, 1993
4. Wang DY, Clement P, Derde MP, Kaulman L: Quantitative data of chemical mediators and inflammatory cells in nasal secretions after nasal allergen challenge. Allergologie 10:415, 1993
5. Kozlov V, Markov GI: New method and devices for diagnosis and treatment of paranasal sinusitis. J Jpn Rhinol Soc 30:1-336, 1991
6. Kozlov VS, Markov GI: New method of diagnosis and treatment of paranasal sinusitis with the application of sinus-catheters 'YAMIK'. Vestnik Otorhinolaryngol 4:32-35, 1993
7. Einstein ER: Proteins of the Brain and CSF in Health and Disease. Illinois USA: Charles C Thomas Publisher 1982
8. Lowry OU, Rosenbrough NJ, Farr AZ, Randall K: Protein measurement with folin phenol reagent. J Biol Chem 1:193 and 265-276, 1951